Abstract
Background: Tabelecleucel (Tab-cel) is an investigational, off-the-shelf, allogenic Epstein-Barr virus (EBV)-specific T-cell immunotherapy. Tab-cel has shown clinical activity in patients with EBV + post-transplant lymphoproliferative disease (PTLD). Previously we have shown that the process for generating tab-cel from healthy donors results in a net amplification of EBV-target responsive T-cell clonality, and that upon activation, tab-cel demonstrates polyfunctionality associated with the secretion of effector and chemoattractive cytokines.
Objective: We aim to comprehensively profile tab-cel through immunophenotype, TCR repertoire by analyzing overall repertoire overlap across lots, and TCR sequence homology (GLIPH 2.0 algorithm), cytokine polyfunctionality (PF), and differential gene expression patterns (GEP) between resting and TCR-MHC-driven EBV antigen stimulation states to replicate intrinsic effector responses associated with EBV + disease engagement.
Methods: Immunophenotyping was performed using targeted FACS activation profiling (CD25/CD69), and 40-plex CyTOF. PF response and cytokine profiles were evaluated using the IsoLight single-cell PF strength assay. TCR repertoires were assessed using TCRβ immunoSEQ, and the GLIPH 2.0 algorithm was utilized to cluster TCRs that are predicted to bind the same MHC-restricted peptide antigen. GEP were evaluated using a custom Nanostring panel consisting of 333 T-cell lineage gene targets.
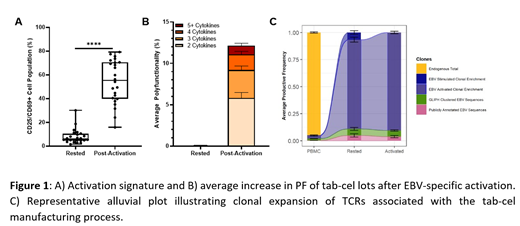
Results: Baseline control activation levels of 7.9±1.3% increased specifically to 54.3±3.7% post-activation with EBV + targets (Figure 1A). Baseline PF was 0.54±0.14%, and upon EBV-specific activation, product cells demonstrated an average PF of 12.1±1.5%, demonstrating a 22.7-fold average increase (Figure 1B).The cytokine profile for activated tab-cel lots is primarily comprised of effector and chemoattractive cytokines including IFNγ and MIP1β. Baseline TCR repertoires of the initial donor peripheral T-cells are highly diverse; however, the tab-cel manufacturing process effectively amplified and enriched for EBV-specific TCRs that correspond back to a starting frequency of 2.6±0.58% of the initial donor TCR repertoire (Figure 1C). Notably, cross comparison of tab-cel-enriched TCRs against publicly available databases (VDJdb, McPas-TCR) identified previously curated EBV-specific TCRβ sequences as a component of the expanded repertoire. Additionally, using the GLIPH 2.0 clustering algorithm we were able to identify previously unannotated TCR sequences that clustered with known EBV-specific TCRβ sequences. The tab-cel post-activation GEP revealed associations with T-cell activation and polyfunctionality. The CD4/CD8 composition of a subset of tab-cel lots was analyzed: the average CD4:CD8 ratio of 0.25, with an average of CD8+ T-cells comprising 73% of the product. An extended immunophenotyping by CyTOF is currently being completed and will be reported at the time of presentation.
Conclusions: In this expanded analysis we again demonstrate that the process for generating tab-cel from healthy donors enriches for known EBV-specific clones and results in a net amplification of EBV-target responsive T-cell clonality. Additionally, utilization of the GLIPH 2.0 clustering algorithm has led to the identification of novel putative EBV-specific TCR sequences that are enriched through the tab-cel manufacturing process. Upon activation, tab-cel exhibits a robust multifactorial activation signaling and demonstrates PF associated with secretion of effector and chemoattractive cytokines. Gene expression profiling of activated tab-cel lots highlights a conserved activation gene signature that is associated with post-activation PF. Lastly, these above analyses are being leveraged to perform correlation studies of immunophenotyped to post-activation PF, TCR repertoire characteristics, and GEP. These data support that the process for generating tab-cel from healthy donor PBMCs leads to the enrichment for EBV-specific T-cell clones that are capable of becoming activated upon stimulation with consistent final product characteristics.
Benoun: Atara Biotherapeutics: Current Employment. Ruiz: Atara Biotherapeutics: Current Employment. Widmann: Atara Biotherapeutics: Current Employment. Jehng: Atara Biotherapeutics: Current Employment. Spindler: Atara Biotherapeutics: Current Employment. Abraham: Atara Biotherapeutics: Current Employment. Minne: Atara Biotherapeutics: Consultancy. Tracy: Atara Biotherapeutics: Current Employment. Munson: Atara Biotherapeutics: Current Employment. Thota: Atara Biotherapeutics: Current Employment. Wang: Atara Biotherapeutics: Current Employment. Chuan: Atara Biotherapeutics: Current Employment. Yedwabnick: Atara Biotherapeutics: Current Employment. Dubovsky: Atara Biotherapeutics: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal